Understanding the Atomic Radius Trend: Exploring the Size Matters of Elements
In the large and intricate realm of chemistry, the periodic table stands as a cornerstone, offering a structured framework to recognize the houses and behaviors of factors. Among the myriad characteristics that define a detail, its atomic radius holds a pivotal function, influencing its chemical and physical residences. Delving into the atomic radius fashion unveils a fascinating narrative of size dynamics across the periodic desk, elucidating the underlying standards governing atomic shape and bonding.
At its center, the atomic radius signifies the gap from the nucleus to the outermost electron shell of an atom. This parameter, at the same time as seemingly straightforward, consists of profound implications for a detail’s reactivity, bond formation, and typical behavior in chemical reactions. Understanding the atomic radius fashion necessitates an adventure through the periodic table, exploring how atomic size evolves across durations and corporations.
Periodic Trends: Unveiling Size Patterns
Within the periodic desk, elements are organized based totally on their atomic range, which denotes the variety of protons inside the nucleus. The arrangement of elements into periods and businesses exhibits awesome tendencies in atomic radius as one traverses the table.
Across Periods: Contrasting Trends
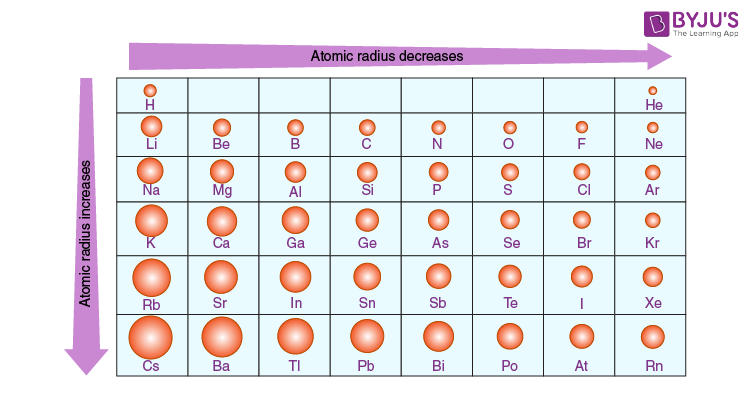
Moving from left to proper throughout a duration, atomic radius reveals a conspicuous fashion of contraction. This phenomenon called the periodic trend of reducing atomic radius, arises often because of the increasing nuclear rate skilled using electrons in the same primary electricity stage. As the number of protons in the nucleus increases, the undoubtedly charged nucleus exerts a stronger pull on the negatively charged electrons, resulting in a tighter electron cloud and a lower atomic radius.
For instance, remember the transition from lithium (Li) to neon (Ne) in Period 2 of the periodic desk. Despite the addition of electrons and energy ranges, the growing nuclear price ends in a slow reduction in atomic radius. This trend continues across the next intervals, exemplifying the regular lower atomic length as one moves across the periodic desk.
Within Groups: Gradual Expansion
Conversely, navigating down a collection inside the periodic desk unravels a contrasting fashion characterized by an increase in atomic radius. This phenomenon, referred to as the periodic trend of growing atomic radius down a group, stems from the addition of electron shells with each descending element. As new electron shells are incorporated, the outermost electrons occupy higher energy levels further from the nucleus, resulting in an expansion of the electron cloud and an increase in atomic length.
For instance, consider Group 1 of the periodic table, which accommodates alkali metals which include lithium (Li), sodium (Na), and potassium (K). As one descends the institution, from lithium to potassium, the atomic radius regularly increases because of the addition of electron shells, despite the simultaneous increase in nuclear price. This trend persists throughout other businesses, highlighting the steady enlargement of atomic size down a column.
Factors Influencing Atomic Radius:
While the periodic developments elucidate overarching patterns in atomic length, several factors contribute to the nuanced versions determined within the periodic table.
Electron Configuration: The distribution of electrons inside an atom’s electron shells drastically affects its atomic radius. Elements with greater electron shells tend to have larger atomic radii because of the improved distance between the outermost electrons and the nucleus.
Nuclear Charge: The number of protons within the nucleus, denoted by way of the atomic quantity, plays a critical role in figuring out the atomic radius. As the nuclear price increases, the enchantment exerted on the outermost electrons intensifies, main to a contraction in atomic size.
Shielding Effect: Electrons in inner strength levels in part guard the outermost electrons from the total effect of the nuclear rate. Thus, factors with more electron shells revel in an extra defensive effect, mitigating the pull of the nucleus and contributing to large atomic radii.
Conclusion of Atomic Radius Trend:
In the problematic tapestry of the periodic table, the atomic radius trend serves as an essential thread, weaving collectively the various houses and behaviors of factors. By unraveling the patterns of atomic length variation across periods and agencies, chemists benefit from useful insights into the underlying concepts governing atomic structure and bonding.
From the innovative contraction located across periods to the sluggish growth of companies, the atomic radius trend embodies the sensitive stability between nuclear rate, electron configuration, and protecting effects. Through meticulous exploration and analysis, scientists retain to deepen their expertise in atomic length dynamics, paving the way for groundbreaking discoveries and improvements inside the realm of chemistry.
Visit for more:timebuissnesnews.com